Abstract
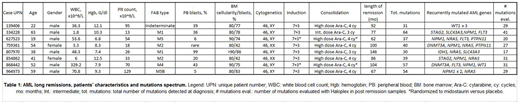
The fraction of patients with cytogenetically normal acute myeloid leukemia (AML) who have long (>5 years) first remissions after chemotherapy alone is estimated to be about 9.1% in young (<60 years) and 1.6% in older (>60 years) patients1; very few intermediate risk patients are therefore "cured" with chemotherapy alone. At this time, it is not yet clear whether these long remission patients are "living with disease", or whether all AML cells have been eliminated. Many people in complete morphologic remission have evidence for persistence of their ancestral and/or founding clones within the first 2-3 years after treatment, but genomic studies of very long first remissions have not yet been described. To determine whether patients with very long first remissions with chemotherapy alone clear their mutations, or are living with persistent disease, we performed an analysis of the mutational landscape and mutation persistence of 8 cases of normal karyotype AML with remissions of >5 years, and who received standard "7+3" induction and high dose cytarabine consolidation (3 to 4 cycles) as their primary therapy. All patients were diagnosed and treated at Washington University in Saint Louis; patient characteristics are summarized in Table 1. The mean age of patients at initial diagnosis was 43.5 years (range 19-63), and the mean time of follow up in first remission is currently 92 months (7.6 years) (range 62-146 months). To the best of our knowledge, none of the patients has relapsed to date. In every case, the day +14 bone marrow demonstrated ablation, and the day +28 bone marrow showed complete morphologic remission.
For each case, we defined the mutational landscape at presentation with enhanced exome sequencing (235x coverage of the entire exome, and ~1008x coverage of recurrently mutated AML genes, as defined in the TCGA AML study). Each case had one or more recognized AML driver mutations, with a mean of 44 (range 31-64) somatic exome mutations (Table 1). The mutational spectrum at diagnosis was not statistically different from larger cohorts of intermediate risk AML patients with standard outcomes: 2 cases had mutations in DNMT3A, 6 had NPMc, 4 had NRAS, 3 had FLT3, 2 had WT1, 2 had STAG2, 2 had SLC43A3, and 2 had PTPN11 mutations. We were able to create probes for 225 mutations (mean of 28 per case, range 17-41) for analysis on the Haloplex platform; we used this platform to perform deep, error-corrected sequencing in one or more remission samples obtained from the bone marrow or peripheral blood of each patient after more than 1 year of remission. The mean depth of Haloplex coverage for all variants for all 8 samples was 1607x (range: 102-12,538x). Importantly, each sample had at least one AML-specific mutation assayed with a coverage depth greater than 3500x, yielding a sensitivity to detect 1 cell in 1,750 (0.06%). In the remission samples, 7/8 patients demonstrated complete clearance of all mutations in all samples tested, strongly suggesting that all AML cells had been eliminated by standard induction and consolidation therapies. In one case (868442), we detected a persistent ancestral clone in morphologic remission samples, harboring DNMT3AR882H (VAF 10.19% in long remission) and IRS2D106Y mutations (VAF 12.82% in long remission). These data suggest that a small subset of normal karyotype AML patients treated with chemotherapy alone may able to completely eradicate all AML cells. Although the mechanism is not yet clear (increased susceptibility to chemotherapy, immunologic surveillance of neoantigens, etc.), these data suggest that some patients in long remissions after chemotherapy do not have persistent subclinical disease, nor do they retain ancestral clones that could potentially contribute to relapse. In ongoing studies, our aim is to define biomarkers that can be used to prospectively identify this subset of patients, so that they can avoid the risk of allotransplantation in first remission.
Reference:
Vasu, Sumithira et al., Blood Advances 2.13 (2018): 1645-1650. Web. 31 July. 2018.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal